This is because they are hydrocarbons that include mostly nonpolar carbon-carbon or carbon-hydrogen bonds. Non-polar molecules are hydrophobic (“water fearing”), or insoluble in water. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of fats. Lipids also provide insulation from the
Fats: Structure and Function – Lavender + Lab Coats
Genetic Transfer As surprising as it seems, deoxyribonucleic acid (DNA) is technically a set of macromolecules. The nucleic acids (A, T, C, and G) that act as codes for genetic material are made of monomers called nucleotides, which also carry genetic materials. DNA separates during meiosis, or sex cell formation.

Source Image: pubs.acs.org
Download Image
For example, a carbohydrate is a macromolecule that is classified as a polymer because it is made up of repeating monosaccharides, but a fat (lipid) is a macromolecule that cannot be further classified because if you look under the ‘monomers’ column, it is built up by more than one monomer. Hope this helped! 2 comments ( 114 votes) Show more

Source Image: pubs.acs.org
Download Image
SOLVED: Macromolecules Food Example Monomer(s) contained in this food Carbohydrates Lipids Proteins Nucleic Acids DNA (in all foods)
Figure 2.14 These examples show three molecules (found in living organisms) that contain carbon atoms bonded in various ways to other carbon atoms and the atoms of other elements. (a) This molecule of stearic acid has a long chain of carbon atoms. (b) Glycine, a component of proteins, contains carbon, nitrogen, oxygen, and hydrogen atoms.

Source Image: pubs.acs.org
Download Image
Salmon Is An Example Of What 2 Macromolecules
Figure 2.14 These examples show three molecules (found in living organisms) that contain carbon atoms bonded in various ways to other carbon atoms and the atoms of other elements. (a) This molecule of stearic acid has a long chain of carbon atoms. (b) Glycine, a component of proteins, contains carbon, nitrogen, oxygen, and hydrogen atoms.
In the reverse of this reaction, water is used to promote hydrolysis. As a reactant, water cleaves the covalent bond that holds the dimer together. B. As a reactant, water cleaves the covalent bond that holds the dimer together. Water is a product of this dehydration synthesis reaction. C. Water is a product of this dehydration synthesis reaction.
Treading Water: Tire Wear Particle Leachate Recreates an Urban Runoff Mortality Syndrome in Coho but Not Chum Salmon | Environmental Science & Technology
3.1: Synthesis of Biological Macromolecules. 3.2: Carbohydrates. Carbohydrates are, in fact, an essential part of our diet; grains, fruits, and vegetables are all natural sources of carbohydrates. Carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple
Cultured meat – Wikipedia

Source Image: en.wikipedia.org
Download Image
Interspecies Differences in 6PPD-Quinone Toxicity Across Seven Fish Species: Metabolite Identification and Semiquantification | Environmental Science & Technology
3.1: Synthesis of Biological Macromolecules. 3.2: Carbohydrates. Carbohydrates are, in fact, an essential part of our diet; grains, fruits, and vegetables are all natural sources of carbohydrates. Carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple

Source Image: pubs.acs.org
Download Image
Fats: Structure and Function – Lavender + Lab Coats
This is because they are hydrocarbons that include mostly nonpolar carbon-carbon or carbon-hydrogen bonds. Non-polar molecules are hydrophobic (“water fearing”), or insoluble in water. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of fats. Lipids also provide insulation from the

Source Image: lavenderandlabcoats.com
Download Image
SOLVED: Macromolecules Food Example Monomer(s) contained in this food Carbohydrates Lipids Proteins Nucleic Acids DNA (in all foods)
For example, a carbohydrate is a macromolecule that is classified as a polymer because it is made up of repeating monosaccharides, but a fat (lipid) is a macromolecule that cannot be further classified because if you look under the ‘monomers’ column, it is built up by more than one monomer. Hope this helped! 2 comments ( 114 votes) Show more

Source Image: numerade.com
Download Image
Antioxidants | Free Full-Text | Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells
Because of their polymeric nature and their large (sometimes huge!) size, they are classified as macromolecules, big ( macro-) molecules made through the joining of smaller subunits. Lipids are not usually polymers and are smaller than the other three, so they are not considered macromolecules by some sources 1, 2 .
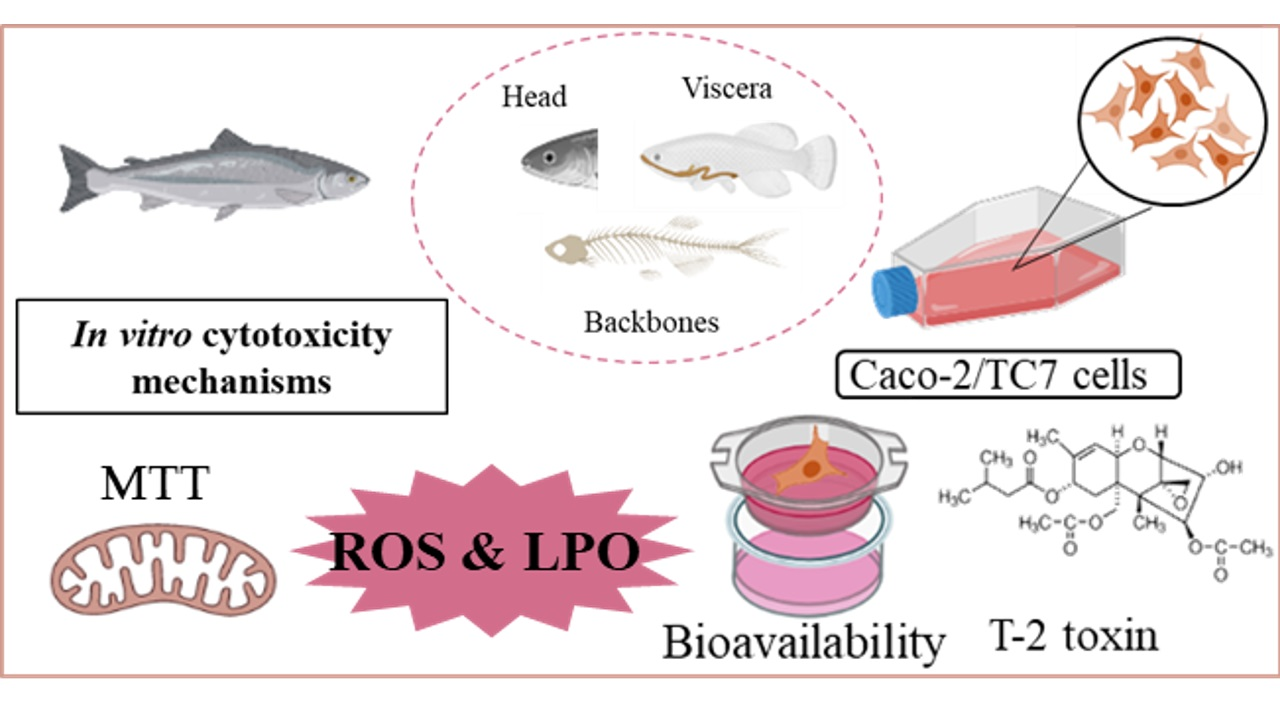
Source Image: mdpi.com
Download Image
CH103 – Chapter 8: The Major Macromolecules – Chemistry
Figure 2.14 These examples show three molecules (found in living organisms) that contain carbon atoms bonded in various ways to other carbon atoms and the atoms of other elements. (a) This molecule of stearic acid has a long chain of carbon atoms. (b) Glycine, a component of proteins, contains carbon, nitrogen, oxygen, and hydrogen atoms.
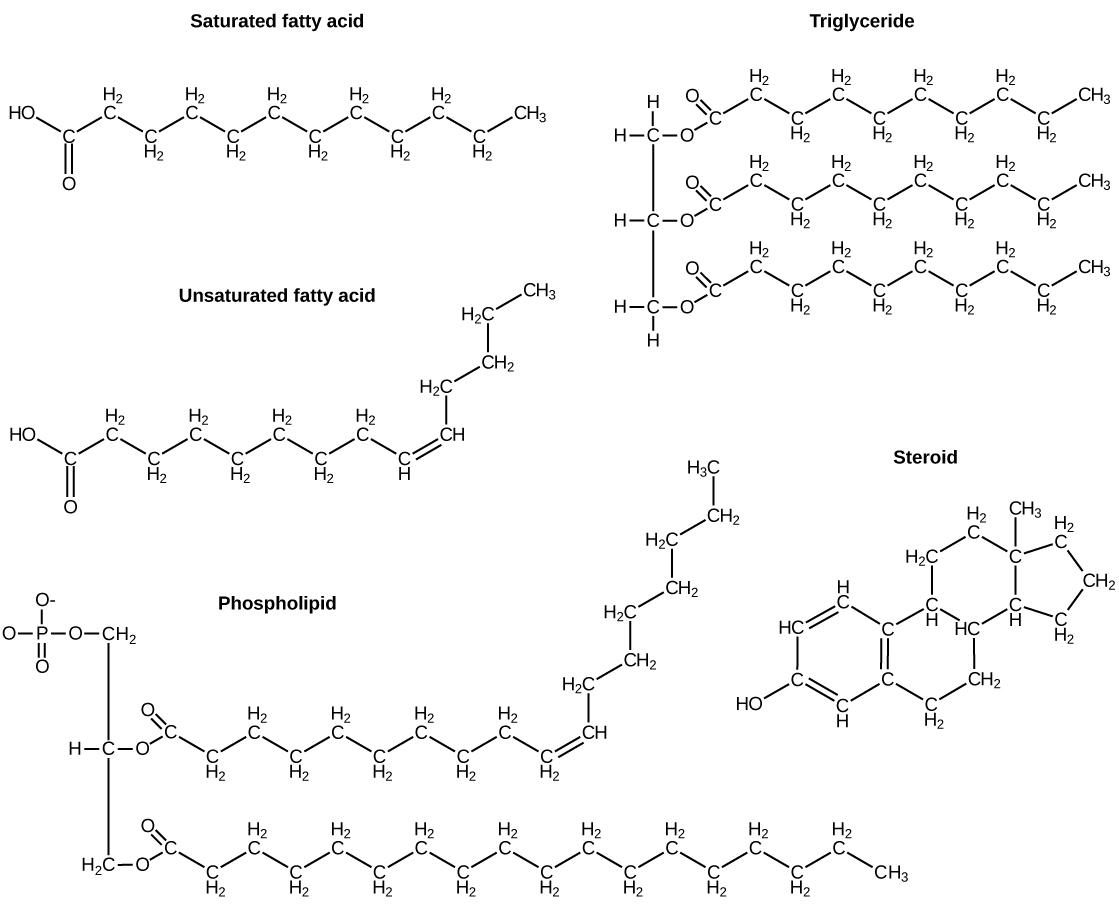
Source Image: wou.edu
Download Image
34 Examples of salmon fillets including high colour index with pink-red… | Download Scientific Diagram
In the reverse of this reaction, water is used to promote hydrolysis. As a reactant, water cleaves the covalent bond that holds the dimer together. B. As a reactant, water cleaves the covalent bond that holds the dimer together. Water is a product of this dehydration synthesis reaction. C. Water is a product of this dehydration synthesis reaction.

Source Image: researchgate.net
Download Image
Interspecies Differences in 6PPD-Quinone Toxicity Across Seven Fish Species: Metabolite Identification and Semiquantification | Environmental Science & Technology
34 Examples of salmon fillets including high colour index with pink-red… | Download Scientific Diagram
Genetic Transfer As surprising as it seems, deoxyribonucleic acid (DNA) is technically a set of macromolecules. The nucleic acids (A, T, C, and G) that act as codes for genetic material are made of monomers called nucleotides, which also carry genetic materials. DNA separates during meiosis, or sex cell formation.
SOLVED: Macromolecules Food Example Monomer(s) contained in this food Carbohydrates Lipids Proteins Nucleic Acids DNA (in all foods) CH103 – Chapter 8: The Major Macromolecules – Chemistry
Because of their polymeric nature and their large (sometimes huge!) size, they are classified as macromolecules, big ( macro-) molecules made through the joining of smaller subunits. Lipids are not usually polymers and are smaller than the other three, so they are not considered macromolecules by some sources 1, 2 .